Background
Glofitamab is a bispecific monoclonal antibody that targets CD20 and CD3, with a unique 2:1 (bivalency for CD20; monovalency for CD3) configuration. In the pivotal trial leading to regulatory approval 1, glofitamab demonstrated remarkable efficacy in patients with relapsed or refractory diffuse large B cell lymphoma (DLBCL). Objective responses were observed in 80% of patients, with 39% achieving complete response. Additionally, complete response was maintained in 78% of patients at 12 months, suggesting that the response might be durable. Most grade 3 or 4 adverse events were hematologic in nature and generally manageable.
Despite its efficacy in clinical trials, the real-world safety and efficacy of glofitamab have not been extensively studied, particularly in patient groups such as those with prior exposure to hepatitis B virus (i.e. occult HBV infection, as evidenced by positive anti-HBV core antigen antibody). Here, we report a single-institution experience of glofitamab in Chinese patients with relapsed or refractory DLBCL, with one-third of them having occult HBV infection.
Methods
This is a retrospective study to evaluate the safety and efficacy of glofitamab in Chinese patients with relapsed or refractory DLBCL. Patients were enrolled through a compassionate program and were required to have failed at least three prior lines of therapy. Patients with active central nervous system lymphoma or active HBV infection (defined as detectable serum hepatitis B surface antigen) were ineligible for treatment. Glofitamab was administered according to the published protocol, with obinutuzumab given on the first day to mitigate the severity of cytokine release syndrome (CRS). Patients with occult HBV infection were given antiviral prophylaxis with entecavir. Positron emission tomography/computerized tomography (PET-CT) was performed after at least two cycles of treatment for response evaluation. Response assessment was made according to published criteria 2. Survival was analyzed using Kaplan-Meier method. Statistical calculations were performed with SPSS Statistics version 28.
Results
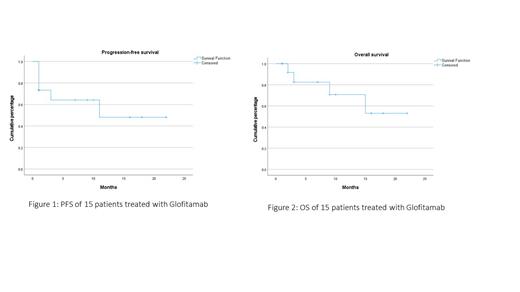
Nine men and six women at a median age of 60 (range: 41-83 years) were treated. Underlying diseases were DLBCL (N=12;80%); transformed follicular lymphoma (N=1, 7%); transformed marginal zone lymphoma (N=1, 7%); and high-grade B-cell lymphoma (N=1, 7%). Other relevant features included staging (I, N=1, 7%; II, N=1, 7%; IV, N=13, 87%); cell of origin (germinal centre B-cell: N=7, 47%; non-germinal centre B-cell: N=7, 47%; not available: N=1, 7%); double-expressor status (positive: N=9, 60%; negative: N=1, 7%; unknown: N=5, 33%); double-hit status(positive: N=3, 20%; negative: N=3, 20%;, unknown: N=9, 60%), prior lines of therapy (median: 6, range: 3-8); prior chimeric receptor T-cell therapy (N=7, 47%); prior autologous transplant (N=4, 27%); elevated serum lactate dehydrogenase (N=9, 60%), and occult HBV infection (N=5, 33%). Eleven patients had a response assessment, showing complete response N=6, 55%), partial response (N=1, 9%) and no response/progressive disease (N=4, 36%). At a median follow-up of 7 months (range 1-22 months) (Figure 1), the median progression-free survival (PFS) was 12 months and the median overall survival (OS) was 15 months(Figure 2). Adverse events included >= grade 3 haematological (anaemia: N=7, 47%; neutropenia: N=8, 53%; thrombocytopenia: N=5, 33%); CRS (grade 1: N=4, 27%; grade 2: N=3, 20%); and cytomegalovirus retinitis (N=1; 6%).
Conclusion
Glofitamab showed similar efficacy in real-world patients compared with published results. It was safe in patients with occult HBV infection given antiviral prophylaxis.
Reference:
1. Dickinson MJ, Carlo-Stella C, Morshhauser F, et al. N Engl J Med 2022; 387:2220-2231
2. Cheson BD, Fisher LI, Barrington SF, et al. J Clin Oncol 2014; 32: 3059-3067
Disclosures
Tse:Merck, Sharp and Dohme: Other: MSD supported author's study abstract ID#184325, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal